SAMPLE LAB REPORT

4.5. How to prepare a copper(II) sulfate solution?
The compound may be available in a hydrated form or at a different concentration or purity.
The anhydrous copper(II) sulfate (cupric sulfate), CuSO4, is a greenish white amorphous powder. It is toxic by ingestion and a strong irritant so care should be taken in handling. When copper(II) sulfate is obtained by crystallization from a water solution, however, five molecules of water are present for each molecule of copper(II) sulfate. The important thing to remember is that the formula weight listed on the bottle of such a compound includes the mass of the water.
The Roman numeral II relates the charge of the copper in this compound is plus 2.
Problem: How many grams of copper(II) sulfate pentahydrate (CuSO4×5H2O) would you need to dissolve in water to produce 1 liter of a 20 % aqueous solution of copper(II) sulfate?
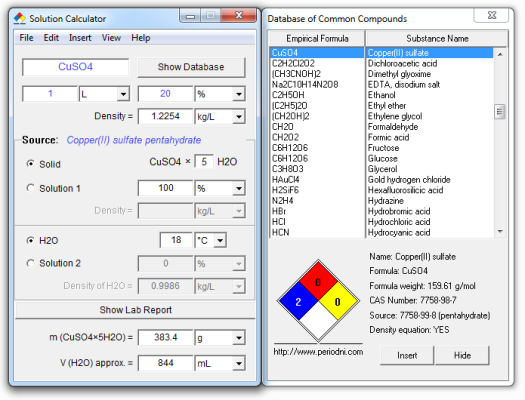
== SolCalc - Laboratory Report ==
September 03, 2012 13:16:44
COPPER(II) SULFATE
w(CuSO4) = 20 %
=======================
To prepare 1 L of a 20 % solution of copper(II) sulfate we have to dissolve
383.399 g of CuSO4×5H2O in deionized or distilled water, and then dilute the
solution to a final volume of 1 L with deionized (distilled) water.
SOURCE:
-------------
Name: Copper(II) sulfate pentahydrate
Formula: CuSO4×5H2O
Formula weight: 249.69 g/mol
CAS Number: 7758-99-8
NFPA: Health 2, Flammability 0, Instability 0, Special
CALCULATION:
-------------
The key concept is that the amount of solute in the desired solution must be
equal to the amount of solute.
Before we make any calculations we have to make sure that we only use one
system and one unit of measurement. DO NOT mix measurement systems and units.
Desired solution:
V0 = 1 L
w0 = 20 % = 20 * 1/100 = 0.2
d0 = 1.22542 kg/L * (1 g/L)/(0.001 kg/L) = 1225.42 g/L
--------------------------------
First, we calculate the concentration of the desired solution
c0 = d(solution) * w(CuSO4) / M(CuSO4)
c0 = 1225.42 g/L * 0.2 / 159.609 g/mol
c0 = 1.53553 mol/L
Now that we know the concentration of the desired solution, we can calculate
the needed mass of the CuSO4×5H2O needed. Note that the available form of
copper(II) sulfate is the hydrated salt.
m(CuSO4×5H2O) = c(CuSO4) * V(solution) * M(CuSO4×5H2O)
m(CuSO4×5H2O) = 1.53553 mol/L * 1 L * 249.685 g/mol
m(CuSO4×5H2O) = 383.4 g
PROCEDURE:
-------------
Weigh the calculated amount of substance in a clean dry weighing bottle.
Transfer the weighed substance into a beaker or Erlenmeyer flask in case the
solid must be heated or crushed in order to be dissolved. Add approximately
half of needed deionized water to the vessel containing the solid and using a
glass rod, stir the contents. After the solid is completely dissolved, pour the
liquid into the clean volumetric flask using a funnel and use the solvent to
rinse all of the solution from the vessel into the flask.
Exceptionally, if the substance is known to be very soluble, the solute may be
added directly to the flask. NEVER heat a solution in a volumetric flask.
Allow the solution to reach room temperature because a volumetric flask is only
accurate at the temperature at which it has been calibrated (usually 20 °C).
Very carefully fill the flask to the mark on the neck of the flask, using a
dropping pipette to add the last few milliliters of liquid. Mix your solution
thoroughly, by inverting the flask and shaking. NEVER hold large volumetric
flasks by the neck alone - provide support at the bottom.
Transfer the prepared solution to a clean, dry storage bottle and label it.
NEVER store solutions in a volumetric flask.
SAFETY NOTES:
-------------
- When making chemical solutions, always use the appropriate safety equipment.
- All chemicals that you are unfamiliar with should be treated with extreme
care and assumed to be highly flammable and toxic.
DISCLAIMER:
-------------
Use SolCalc at your own risk! If you don't understand the results, DON'T use
them.
=======================
https://www.periodni.com
Citing this page:
Generalic, Eni. "SolCalc Help: Preparing CuSO4 solution." EniG. Periodic Table of the Elements. KTF-Split, 27 Oct. 2022. Web. {Date of access}. <https://www.periodni.com/enig/solcalc_help/preparing_copper_sulfate.html>.
Articles and tables
- Periodic table
- Online calculators
- Scientific calculator for chemists
- Gas laws calculator
- Molar mass calculator
- Angle converter
- Roman numerals converter
- Number systems converter
- Preparation of solutions
- Labeling of chemical containers
- Oxidation numbers calculator
- ARS method
- Oxidation number change method
- Ion-electron method
- Gauss elimination method
- Memory game
- Find the pairs
- Articles and tables
- Chemistry
- List of abbreviations and acronyms
- Crystal systems and Bravais lattices
- GHS - Hazard pictograms
- NFPA 704 Hazard Diamond
- Fundamental physical constants
- Solubility product constants
- SI - International System of Units
- Composition of mixtures and solutions
- Stoichiometric calculations
- Chlorinity and salinity of seawater
- Rare earth elements (REE)
- Ecology
- Web design
- Chemistry dictionary
- Chemistry
- Downloads
- ≡ Menu
